|
This section contains 7,149 words (approx. 24 pages at 300 words per page) |
What Is Acid Rain?
Acid rain is the common name for acidic deposits that fall to Earth from the atmosphere. The term was coined in 1872 by English chemist Robert Angus Smith to describe the acidic precipitation in Manchester, England. Today scientists study both wet and dry acidic deposits. Although there are natural sources of acid in the atmosphere, acid rain is primarily caused by emissions of sulfur dioxide (SO2) and nitrous oxide (N2O) from electric utilities burning fossil fuels, especially coal. These chemicals are converted to sulfuric acid and nitric acid in the atmosphere and can be carried by the winds for many miles from where the original emissions took place. (See Figure 7.1.)
Wet deposition occurs when the acid falls in rain, snow, or ice. Dry deposition is caused by very tiny particles (or particulates) in combustion emissions. They may stay dry as they fall or pollute cloud water and precipitation. Moist deposition occurs when the acid is trapped in cloud or fog droplets. This is most common at high altitudes and in coastal areas. Whatever its form, acid rain can create dangerously high levels of acidic impurities in water, soil, and plants.
Measuring Acid Rain
The acidity of any solution is measured on a potential hydrogen (pH) scale numbered from zero to 14, with a pH value of seven considered neutral. Values higher than seven are considered more alkaline or basic (the pH of baking soda is eight); values lower than seven are considered acidic (the pH of lemon juice is two). The pH scale is a logarithmic measure. This means that every pH change of one is a ten-fold change in acid content. Therefore, a decrease from pH seven to pH six is a ten-fold increase in acidity; a drop from pH seven to pH five is a 100-fold increase in acidity; and a drop from pH seven to pH four is a 1,000-fold increase. (See Figure 7.2.)
Pure, distilled water has a neutral pH of seven. Normal rainfall has a pH value of about 5.6. It is slightly acidic because it accumulates naturally occurring sulfur oxides (SO5) and nitrogen oxides (NO5) as it passes through the atmosphere. Acid rain has a pH of less than 5.6.
Figure 7.3 shows the average rainfall pH measured during 2002 at various field laboratories around the country by the National Atmospheric Deposition Program, a cooperative project between many state and federal government agencies and private entities. Rainfall was most acidic in the Mid-Atlantic states, particularly New York, Pennsylvania, Maryland, Ohio, West Virginia, and in portions of western Virginia, North Carolina, eastern Tennessee, and Kentucky. Unfortunately, the areas with lowest rainfall pH contain some of the country's most sensitive natural resources—the Appalachian Mountains, Adirondack Mountains, Chesapeake Bay, and Great Smoky Mountains National Park.
Sources of Sulfate and Nitrate in the Atmosphere
Natural Sources
Natural sources of sulfate in the atmosphere include ocean spray, volcanic emissions, and readily oxidized hydrogen sulfide released from the decomposition of organic matter found in the Earth. Natural sources of nitrogen or nitrates include NO5 produced by microorganisms in soils, by lightning during thunderstorms, and by forest fires. Scientists generally speculate that one-third of the sulfur and nitrogen emissions in the United States comes from these natural sources (this is a rough estimate as there is no way to measure natural emissions as opposed to those that are manmade.)
Sources Caused by Human Activity
The primary anthropogenic (human-caused) contributors to acid rain are SO2 and NO5, resulting from the burning of fossil fuels, such as coal, oil, and natural gas.
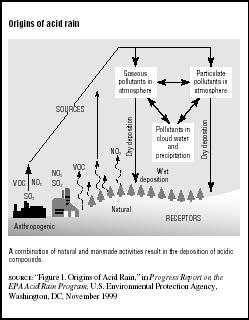 FIGURE 7.1
FIGURE 7.1Origins of acid rain
Figure 5.15 in Chapter 5 shows the breakdown of U.S. SO2 emissions by source from 1983 to 2002. Fuel combustion by fossil-fueled electric utilities historically has been by far the greatest source of these emissions, accounting for 85 percent of them in 2002. Lesser sources included transportation vehicles and industrial processes.
NO5 emission sources are shown in Figure 5.6 in Chapter 5. Transportation vehicles are the primary source, accounting for 56 percent of the total in 2002. Fuel combustion in power plants is another major source, accounting for 37 percent of the total. Emissions by industry and miscellaneous sources (for example, agriculture) accounted for only 7 percent of the total. Agricultural emissions of nitrogen compounds are due to windblown fertilizers.
Nitrogen pollution of waters has historically been blamed on surface runoff from fertilizer, animal waste, sewage, and industrial waste. Although these are still significant causes, scientists have come to believe that airborne nitrates account for one-fourth of all nitrogen, the second most prevalent cause after fertilizers. Scientists also blame ammonia emissions, which come largely from agricultural activities such as manure handling and fertilizing, for contributing to acid rain. According to the U.S. Geological Survey (USGS), ammonium levels in precipitation increased throughout the 1990s across most of the country. The average increase was 24 percent.
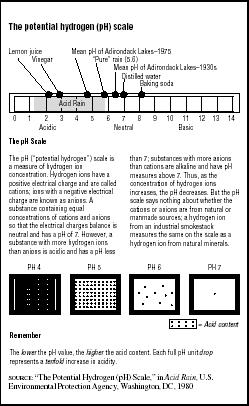 FIGURE 7.2
FIGURE 7.2The potential hydrogen (pH) scale
Natural Factors That Affect Acid Rain Deposition
Air Movement
Several factors contribute to the impact of acid rain on an area. Transport systems—primarily the movement of air—distribute acid emissions in definite patterns around the planet. The movement of air masses transports emitted pollutants many miles, during which the pollutants are transformed into sulfuric and nitric acid by mixing with clouds of water.
In the United States a typical transport pattern occurs from the Ohio River Valley to the northeastern United States and southeastern Canada, as prevailing winds tend to move from west to east and from south to north. About one-third of the total sulfur compounds deposited over the eastern United States originates from sources in the Midwest more than 300 miles away.
Climate
In drier climates, such as those of the western United States, windblown alkaline dust moves more freely through the air and tends to neutralize atmospheric acidity. The effects of acid rain can be greatly reduced by the presence of basic (also called alkali) substances. Sodium, potassium, and calcium are examples of basic chemicals. When a basic and an acid chemical come into contact, they react chemically and neutralize each other. On the other hand, in more humid climates where there is less dust, such as along the eastern seaboard, precipitation is more acidic.
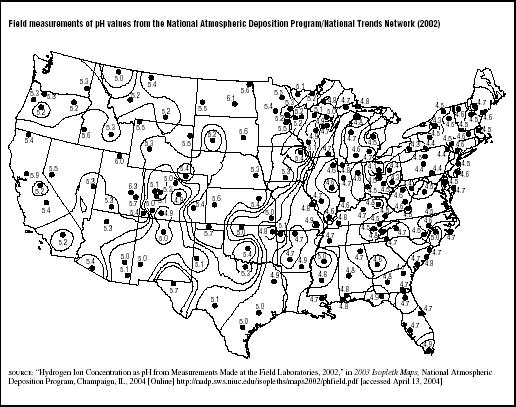 FIGURE 7.3
FIGURE 7.3Field measurements of pH values from the National Atmospheric Deposition Program/National Trends Network (2002)
Topography/Geology
Areas most sensitive to acid rain contain hard, crystalline bedrock and very thin surface soils. When no alkaline-buffering particles are in the soil, runoff from rainfall directly affects surface waters, such as mountain streams. In contrast, a thick soil covering or soil with a high buffering capacity, such as flat land, neutralizes acid rain better. Lakes tend to be most susceptible to acid rain because of low alkaline content in lake beds. A lake's depth, its watershed (the area draining into the lake), and the amount of time the water has been in the lake are also factors.
The lakes and forests in and near the Adirondack Mountains in upstate New York are an example of what occurs in areas that do not have carbonate rock to quickly neutralize acid. Approximately half the lakes above the altitude of 2,000 feet have a pH of less than 5.0. Ninety percent of these lakes contain no aquatic life.
The states bordering and east of the Mississippi River contain approximately 17,000 lakes and 112,000 miles of streams. An estimated 25 percent of the land contains soil and bedrock that allow acidity to travel through underground water to these lakes and streams. Approximately half of these bodies of water have such a limited ability to neutralize acid that acid-laden pollutants will eventually cause acidification.
Effects of Acid Rain on Our Environment
In nature, the combination of rain and oxides is part of a natural balance that nourishes plants and aquatic life. However, when the balance is upset, the results to the environment can be harmful and destructive. (See Table 7.1.)
Table 7.1
Effect of acid rain on human health and selected ecosystems and anticipated recovery benefits
| Human health and ecosystem | Effects | Recovery benefits |
| Human health | In the atmosphere, sulfur dioxide and nitrogen oxides become sulfate and nitrate aerosols, which increase morbidity and mortality from lung disorders, such as asthma and bronchitis, and impacts to the cardiovascular system. | Decrease emergency room visits, hospital admissions, and deaths. |
| Surface waters | Acidic surface waters decrease the survivability of animal life in lakes and streams and in the more severe instances eliminate some or all types of fish and other organisms. | Reduce the acidic levels of surface waters and restore animal life to the more severely damaged lakes and streams. |
| Forests | Acid deposition contributes to forest degradation by impairing trees' growth and increasing their susceptibility to winter injury, insect infestation, and drought. It also causes leaching and depletion of natural nutrients in forest soil. | Reduce stress on trees, thereby reducing the effects of winter injury, insect infestation, and drought, and reduce the leaching of soil nutrients, thereby improving overall forest health. |
| Materials | Acid deposition contributes to the corrosion and deterioration of buildings, cultural objects, and cars, which decreases their value and increases costs of correcting and repairing damage. | Reduce the damage to buildings, cultural objects, and cars, and reduce the costs of correcting and repairing future damage. |
| Visibility | In the atmosphere, sulfur dioxide and nitrogen oxides form sulfate and nitrate particles, which impair visibility and affect the enjoyment of national parks and other scenic views. | Extend the distance and increase the clarity at which scenery can be viewed, thus reducing limited and hazy scenes and increasing the enjoyment of national parks and other vistas. |
| SOURCE: "Appendix I: Effect of Acid Rain on Human Health and Selected Ecosystems and Anticipated Recovery Benefits," in Acid Rain: Emissions Trends and Effects in the Eastern United States, U.S. General Accounting Office, Washington, DC, March 2000 | ||
Aquatic Systems
Although pH levels vary considerably from one body of water to another, a typical pH range for the lakes and rivers in the United States is six to eight.
Low pH levels kill fish eggs, frog eggs, and fish food organisms. The degree of damage depends on several factors, one of which is the buffering capacity of the watershed soil—the higher the alkalinity, the more slowly the lakes and streams acidify. The exposure of fish to acidified freshwater lakes and streams has been intensely studied since the 1970s. Scientists distinguish between sudden shocks and chronic (long-term) exposure to low pH levels.
Sudden, short-term shifts in pH levels result from snowmelts, which release acidic materials accumulated during the winter, or sudden rainstorms that can wash residual acid into streams and lakes. The resulting acid shock can be devastating to fish and their ecosystems. At pH levels below 4.9, damage occurs to fish eggs. At acid levels below 4.5, some species of fish die. Below pH 3.5, most fish die within hours. (See Table 7.2.)
Mountainous streams in New York, North Carolina, Pennsylvania, Tennessee, and Arkansas have shown an acidity during rainstorms and snowmelts of three to 20 times that experienced during the rest of the year. Because many species of fish hatch in the spring, even mild increases in acidity can harm or kill the new life. Temporary increases in acidity also affect insects and other invertebrates, such as snails and crayfish, on which the fish feed.
Gradual decreases of pH levels over time affect fish reproduction and spawning. Moderate levels of acidity in water can confuse a salmon's sense of smell, which it uses to find the stream from which it came. Atlantic salmon are unable to find their home streams and rivers because of acid rain. In addition, excessive acid levels in female fish cause low amounts of calcium, thereby preventing the production of eggs. Even if eggs are produced, their development is often abnormal. Over time the fish population decreases while the remaining fish population becomes older and larger.
Increased acidity can also cause the release of aluminum and manganese particles stored in a lake or river bottom. High concentrations of these metals are toxic to fish.
In 1988 the Environmental Defense Fund (EDF), an environmental watch group, sounded one of the first alarms that the coastal waters of the eastern United States were receiving large inputs of nitrogen. The nitrogen led to an excessive growth of algae on the surface of the water. This in turn resulted in the loss of oxygen and light to the water and the long-term decline of marine life. The EDF concluded that the major sources of the nitrogen were human activities—the runoff of fertilizer, animal waste from farms, and discharge from sewage treatment plants and industrial facilities. Researchers noted that the significant decline of the Chesapeake Bay and other estuaries could also be attributed to the increase in NO5 from automobiles and electric power plants, along with toxic chemicals, pesticides, and wetland destruction.
Table 7.2
Generalized short-term effects of acidity on fish
| pH range | Effect |
| 6.5–9 | No effect |
| 6.0–6.4 | Unlikely to be harmful except when carbon dioxide levels are very high (1000 mg I 1) |
| 5.0–5.9 | Not especially harmful except when carbon dioxide levels are high (20 mg I 1) or ferric ions are present |
| 4.5–4.9 | Harmful to the eggs of salmon and trout species (salmonids) and to adult fish when levels of Ca2, Na and Cl are low |
| 4.0–4.4 | Harmful to adult fish of many types which have not been progressively acclimated to low pH |
| 3.5–3.9 | Lethal to salmonids, although acclimated roach can survive for longer |
| 3.0–3.4 | Most fish are killed within hours at these levels |
| SOURCE: "Generalized Short-Term Effects of Acidity on Fish," in National Water Quality Inventory: 1998 Report to Congress, U.S. Environmental Protection Agency, Washington, DC, June 2000 | |
During the 1990s acidic and polluted waters caused the disappearance of many aquatic species, leaving gaping holes in the food chain and diminishing the biological balance and diversity that keeps Earth genetically healthy. According to the American Fisheries Society and the Environmental Protection Agency (EPA), many species of freshwater fish have become extinct since the late 1970s, and additional species have become endangered, threatened, or listed as "of special concern" for their ultimate survival.
Soil and Vegetation
Acid rain is believed to harm vegetation by changing soil chemistry. Soils exposed to acid rain can gradually lose valuable nutrients, such as calcium, magnesium, and potassium, and become too concentrated with dissolved inorganic aluminum, which is toxic to vegetation. Long-term changes in soil chemistry may have already affected sensitive soils, particularly in forests. Forest soils saturated in nitrogen cannot retain other nutrients required for healthy vegetation. Subsequently, these nutrients are washed away. The EPA reports that nitrogen saturation has already been found in a number of regions, including northeastern forests, the Colorado Front Range, and mountain ranges near Los Angeles, California. The same effects have been reported in Canada and Europe. Nutrient-poor trees are more vulnerable to climatic extremes, pest invasion, and the effects of other air pollutants, such as ozone.
Some researchers believe that acid rain disrupts soil regeneration, which is the recycling of chemical and mineral nutrients through plants and animals back to the Earth. They also believe acids suppress decay of organic matter, a natural process needed to enrich the soils. Valuable nutrients like calcium and magnesium are normally bound to soil particles and are, therefore, protected from being rapidly washed into groundwater. Acid rain, however, may accelerate the process of breaking these bonds to rob the soil of these nutrients. This, in turn, decreases plant uptake of vital nutrients. (See Figure 7.4.)
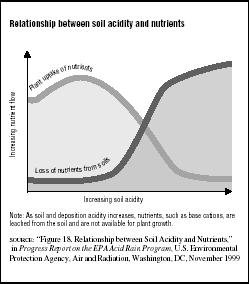 FIGURE 7.4
FIGURE 7.4Relationship between soil acidity and nutrients
Acid deposition can cause leafy plants such as lettuce to hold increased amounts of potentially toxic substances like the mineral cadmium. Research has also found a decrease in carbohydrate production in the photosynthesis process of some plants exposed to acid conditions. Research is underway to determine whether acid rain could ultimately lead to a permanent reduction in tree growth, food crop production, and soil quality. Effects on soils, forests, and crops are difficult to measure because of the numerous species of plants and animals, the slow rate at which ecological changes occur, and the complex interrelationships between plants and their environment.
Trees.
The effect of acid rain on trees is influenced by many factors. Some trees adapt to environmental stress better than others; the type of tree, its height, and its leaf structure (deciduous or evergreen) influence how well it will adapt to acid rain. Acid rain may affect trees in at least two ways: in areas with high evaporation rates, acids will concentrate on leaf surfaces; in regions where a dense leaf canopy does not exist, more acid may seep into the Earth to affect the soil around the tree's roots.
Scientists believe that acid rain directly harms trees by leaching calcium from their foliage and indirectly harms them by lowering their tolerance to other stresses. Trees are exposed to many natural threats, including drought, ice storms, invasive species, and forest fires. These stresses, combined with increased air and water pollution, can prove too much for sensitive tree species.
A 1994 joint report of the European Commission and the UNECE surveyed 102,300 trees at 26,000 sampling plots in 35 European countries and found that almost one-quarter of the trees in Europe were defoliated by more than 25 percent. The report showed that forest damage is a problem in virtually all European countries. The most severely affected country was the Czech Republic, where 53 percent of all trees had suffered moderate or severe defoliation or died. The least affected was Portugal, where 7.3 percent of trees were damaged.
In 1998 the National Acid Precipitation Assessment Program (NAPAP) identified forest ecosystems in the United States that are most at risk to acid rain damage due to natural sensitivity and high acid deposition rates. (See Figure 7.5.) The EPA blames acid deposition, along with other pollutants and natural stress factors, for increased death and decline of northeastern red spruce at high elevations (for example, in the Adirondacks) and decreased growth of red spruce in the southern Appalachians. Acid rain is also closely linked to the decline of sugar maple trees in Pennsylvania.
In Soil Calcium Depletion Linked to Acid Rain and Forest Growth in the Eastern United States, the USGS reported in March 1999 that calcium levels in forest soils had declined at locations in ten states in the eastern United States. Calcium is necessary to neutralize acid rain and is an essential nutrient for tree growth. Sugar maple and red spruce trees, in particular, showed reduced resistance to stresses such as insect defoliation and low winter temperatures. Although the specific relationships among calcium availability, acid rain, and forest growth are uncertain, Gregory Lawrence, a scientist and coauthor of the report, speculated: "Acid rain releases aluminum from the underlying mineral soil layer.… The result is that aluminum replaces calcium, and the trees have a harder time trying to get the needed calcium from the soil layer."
In 2001 the Hubbard Brook Research Foundation reported that more than half of large-canopy red spruce trees in the Adirondack Mountains and the Green Mountains had died since the 1960s. Acid rain was considered the primary cause.
According to the EPA, acid rain has also been implicated in impairing the winter hardening process of some trees, making them more susceptible to cold-weather damage. In some trees, the roots are prone to damage because the movement of acidic rain through the soil releases aluminum ions, which are toxic to plants.
One area in which acid rain has been linked to direct effects on trees is from moist deposition via acidic fogs and clouds. The concentrations of acid and SO5 in fog droplets are much greater than in rainfall. In areas of frequent fog, such as London, significant damage has occurred to trees and other vegetation because the fog condenses directly on the leaves.
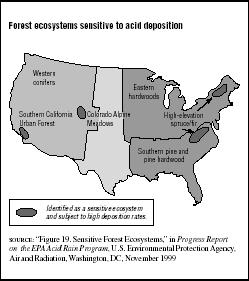 FIGURE 7.5
FIGURE 7.5Forest ecosystems sensitive to acid deposition
The Forest Health Monitoring Program is a joint effort supported by the U.S. Department of Agriculture (USDA) and private and academic entities. The program monitored precipitation pH and the deposition of SO5, nitrate, ammonium, and total nitrogen in U.S. forests from 1979 to 1995. It estimates that nearly half of forest area in the North and just over 20 percent of forest area in the South are covered by relatively high SO5 deposition. Northern forests were much more exposed to nitrate deposition (40 percent) than were southern forests (less than 1 percent). High ammonium deposition was a problem for more than 62 percent of forests in the North, but less than 20 percent of forests in the South.
Birds
Increased freshwater acidity harms some species of migratory birds. Experts believe the dramatic decline of the North American black duck population since the 1950s is due to decreased food supplies in the acidified wetlands. The U.S. Fish and Wildlife Service reports that ducklings in wetlands created by humans in Maryland are three times more likely to die before adulthood if raised in acidic waters.
Acid rain leaches calcium out of the soil and robs snails of the calcium they need to form shells. Because tit-mice and other species of songbirds get most of their calcium from the shells of snails, the birds are also perishing. The eggs they lay are defective—thin and fragile. The chicks either do not hatch or have bone malformations and die.
In 2002 researchers at Cornell University released the results of a large-scale study showing a clear link between acid rain and widespread population declines in a song-bird called the wood thrush. The scientists believe that calcium depletion has had a negative impact on the birds' food source, mainly snails, earthworms, and centipedes. The birds may also be ingesting high levels of metals that are more likely to leach out of overly acidic soils. Declining wood thrush populations were most pronounced in the higher elevations of the Adirondack, Great Smoky, and Appalachian mountains. The researchers warned that acid rain may also be contributing to population declines in other songbird species.
Materials
Investigations into the effects of acid rain on objects such as stone buildings, marble statues, metals, and paints only began in the 1990s. A joint study conducted by the EPA, the Brookhaven National Laboratory, and the Army Corps of Engineers in 1993 found that acid rain was causing $5 billion worth of damage annually in a 17-state region. Two-thirds of the damage was created by pollution whose source was less than 30 miles away. Many of the country's historical monuments and buildings are located in eastern states that have been most hard-hit by acid rain.
Acid rain is suspected, in part, of damaging the Statue of Liberty and the Egyptian pyramids. Examination of the 700-year-old, 37-foot-tall bronze Great Buddha of Kamakura, an important symbol of Japanese culture, shows pock marks and rust stains, the result of acid rain.
New kinds of protective chemicals that adhere to limestone and marble are helping to save some of the world's decomposing monuments from acid rain and other pollutants. These chemicals, called consolidants, were developed in the 1960s in response to widespread water damage to stone buildings in Venice. Among the monuments getting close attention are the Taj Mahal in India; the Parthenon in Athens, Greece; the Lincoln Memorial in Washington, D.C.; and the Alamo in Texas. Experts report, however, that these chemicals have many limitations. They are toxic and difficult to apply, and their effects are only temporary, yet they permanently alter the nature of the stone. Most important is that their long-term effects are uncertain. For those reasons their use was banned on the Acropolis in Athens, Greece.
Automotive Coatings.
Reports of damage to automotive coverings have been increasing. The general consensus within the automobile industry is that the damage is caused by some form of "environmental fallout"—the term used in the automobile industry. Automakers suspect acid rain damage to automobile paint, especially to many newer models that have clear protective overcoats. Chemical analyses of the damaged areas of some car finishes have showed elevated levels of SO5, implicating acid rain.
The auto industry began using clear-coat finishes in the mid-1980s. Although the new high-gloss paints look better, complaints are mounting over marred surfaces, especially on dark-or metallic-colored cars in the northeastern and southeastern United States. Automakers believe that when acid rain falls on autos the moisture evaporates, leaving a permanent blemish caused by sulfuric acid and nitric acid—the composition of acid rain. Some car dealers now offer optional protective sealants at added expense to buyers. Higher-priced cars often include protective sealants in the purchase price.
Human Health
Acid rain has several direct and indirect effects on human health. Particulates are extremely small pollutant particles that can threaten human health. Particulates related to acid rain include fine particles of SO5 and nitrates. These particles can travel long distances and, when inhaled, penetrate deep into the lungs. Studies of death rates across the United States, such as that reported in "Lung Cancer, Cardiopulmonary Mortality, and Long-Term Exposure to Fine Particulate Air Pollution" (Journal of the American Medical Association, March 6, 2002), have found some correlation between elevated mortality levels and high SO5 levels. Acid rain and the pollutants that cause it can lead to the development of bronchitis and asthma in children. Acid rain is also believed to be responsible for increasing health risks to those over the age of 65; those with asthma, chronic bronchitis, and emphysema; pregnant women; and those with histories of heart disease.
The Politics of Acid Rain
Scientific research on acid rain was sporadic and largely focused on local problems until the late 1960s, when Scandinavian scientists began more systematic studies. Acid precipitation in North America was not identified until 1972, when scientists found that precipitation was acidic in eastern North America, especially in northeastern and eastern Canada. In 1975 the First International Symposium on Acid Precipitation and the Forest Ecosystem convened in Columbus, Ohio, to define the acid rain problem. Scientists used the meeting to propose a precipitation-monitoring network in the United States that would cooperate with the European and Scandinavian networks and to set up protocols for collecting and testing precipitation.
In 1977 the Council on Environmental Quality was asked to develop a national acid rain research program. Several scientists drafted a report that eventually became the basis for NAPAP. This initiative eventually translated into legislative action with the Energy Security Act (PL 96-264) in June 1980. Title VII of the Energy Security Act (the Acid Precipitation Act of 1980) produced a formal proposal that created NAPAP and authorized federally financed support.
The first international treaty aimed at limiting air pollution was the UNECE Convention on Long-Range Transboundary Air Pollution, which went into effect in 1983. It was ratified by 38 of the 54 UNECE members, which included not only European countries but also Canada and the United States. The treaty targeted sulfur emissions, requiring that countries reduce emissions 30 percent from 1980 levels—the so-called "30 percent club."
The early acid rain debate centered almost exclusively on the eastern United States and Canada. The controversy was often defined as a problem of property rights. The highly valued production of electricity in coal-fired utilities in the Ohio River Valley caused acid rain to fall on land in the Northeast and Canada. An important part of the acid rain controversy in the 1980s was the adversarial relationship between U.S. and Canadian government officials over emission controls of SO2 and NO2. More of these pollutants crossed the border into Canada than the reverse. Canadian officials very quickly came to a consensus over the need for more stringent controls, while this consensus was lacking in the United States.
Throughout the 1980s the major lawsuits involving acid rain all came from eastern states, and the states that passed their own acid rain legislation were those in the eastern part of the United States.
Legislative attempts to restrict emissions of pollutants were often defeated after strong lobbying by the coal industry and utility companies. Those industries advocated further research for pollution-control technology rather than placing restrictions on utility company emissions.
The Acid Rain Program—Clean Air Act Amendments, Title IV
In 1980 Congress established NAPAP to study the causes and effects of acid deposition. About 2,000 scientists worked with an elaborate multimillion-dollar computer model in an eight-year, $570 million undertaking. In 1988 NAPAP produced an overwhelming 6,000-page report on its findings, including:
- Acid rain had adversely affected aquatic life in about 10 percent of eastern lakes and streams.
- Acid rain had contributed to the decline of red spruce at high elevations by reducing that species' cold tolerance.
- Acid rain had contributed to erosion and corrosion of buildings and materials.
- Acid rain and related pollutants had reduced visibility throughout the Northeast and in parts of the West.
The report concluded, however, that the incidence of serious acidification was more limited than originally feared. The Adirondacks area of New York was the only region showing widespread, significant damage from acid at that time.
Results indicated that electricity-generating power plants were responsible for two-thirds of SO2 emissions and one-third of NO5 emissions. In response, Congress created the Acid Rain Program under Title IV (Acid Deposition Control) of the 1990 Clean Air Act Amendments (PL 101-549).
The goal of the Acid Rain Program is to reduce annual emissions of SO2 and NO5 from electric power plants nationwide. The program set a permanent cap on the total amount of SO2 that could be emitted by these power plants. That cap was set at 8.95 million tons (approximately half the number of tons of SO2 emitted by these plants during 1980). The program also established NO5 emissions limitations for certain coal-fired electric utility plants. The objective of the NO5 program was to achieve and maintain a two-million-ton reduction in NO5 emission levels by the year 2000 compared to the emissions that would have occurred in 2000 if the program had not been implemented.
The reduction was implemented in two phases. Phase 1 began in 1995 and covered 263 units at 110 utility plants in 21 states with the highest levels of emissions. Most of these units were at coal-burning plants in eastern and midwestern states. They were mandated to reduce their annual SO2 emissions by 3.5 million tons. An additional 182 units joined Phase 1 voluntarily, bringing the total of Phase 1 units to 445.
Phase 2 began in 2000. It tightened annual emission limits on the Phase 1 group and set new limits for more than 2,000 cleaner and smaller units in all 48 contiguous states and the District of Columbia.
A New Flexibility in Meeting Regulations
Traditionally, environmental regulation has been achieved by the "command and control" approach, in which the regulator specifies how to reduce pollution, by what amount, and what technology to use. Title IV, however, gave utilities flexibility in choosing how to achieve these reductions. For example, utilities could reduce emissions by switching to low-sulfur coal, installing pollution-control devices called scrubbers, or shutting down plants.
Utilities took advantage of their flexibility under Title IV to choose less costly ways to reduce emissions, such as switching from high-to low-sulfur coal, and they have been achieving sizable reductions in their SO2 emissions. Fifty-five percent of Phase 1 plants opted to switch to low-sulfur coal, 16 percent chose to install scrubbers, and only 3 percent initially planned to purchase allowances (which allow plants to emit extra SO2). Not surprisingly, the market for low-sulfur coal is growing as a result of Title IV, and the market for high-sulfur coal is decreasing.
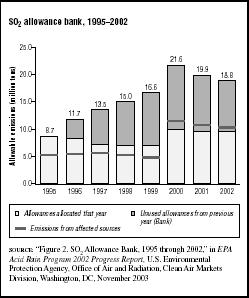 FIGURE 7.6
FIGURE 7.6SO2 allowance bank, 1995–2002
Allowance Trading
Title IV also allows electric utilities to trade allowances to emit SO2. Utilities that reduce their emissions below the required levels can sell their extra allowances to other utilities to help them meet their requirements.
Title IV allows companies to buy, sell, trade, and bank pollution rights. Utility units are allocated allowances based on their historic fuel consumption and a specific emissions rate. Each allowance permits a unit to emit one ton of SO2 during or after a specific year. For each ton of SO2 discharged in a given year, one allowance is retired and can no longer be used. Companies that pollute less than the set standards will have allowances left over. They can then sell the difference to companies that pollute more than they are allowed, bringing them into compliance with overall standards. Companies that clean up their pollution would recover some of their costs by selling their pollution rights to other companies.
The EPA holds an allowance auction each year. The sale offers allowances at a fixed price. This use of market-based incentives under Title IV is regarded by many as a major new method for controlling pollution.
From 1995 to 1998 there was considerable buying and selling of allowances among utilities. Because the utilities that participated in Phase 1 reduced their sulfur emissions more than the minimum required, they did not use as many allowances as they were allocated for the first four years of the program. Those unused allowances could be used to offset SO2 emissions in future years. From 1995 to 1998 a total of 30.2 million allowances were allocated to utilities nationwide; almost 8.7 million, or 29 percent, of the allowances were not used, but were carried over (banked) for subsequent years.
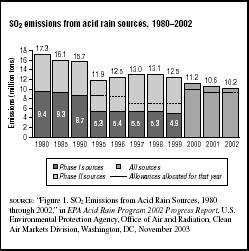 FIGURE 7.7
FIGURE 7.7SO2 emissions from acid rain sources, 1980–2002
Figure 7.6 shows the status of the allowance bank from 1995 through 2002. In 2002 a total of 9.54 million allowances were allocated. Another 9.30 million banked allowances were carried over from previous years. The allowance bank reached a maximum during 2000 and began to decline after that. The EPA expects that the allowance bank will gradually be depleted.
Emissions and Deposition
Each year the EPA publishes a report detailing the progress achieved by the Acid Rain Program. The latest report is titled Acid Rain Program, 2002 Progress Report and was published in November 2003.
The report notes that in 2002 there were 3,208 electric generating units subject to the SO2 provisions of the Acid Rain Program. They emitted 10.2 million tons of SO2 into the air as shown in Figure 7.7. The EPA expects that the 8.95-million-ton annual cap will be achieved by the year 2010. SO2 emissions from sources covered by the program decreased by 41 percent between 1980 and 2002.
The downward trend in SO2 emissions was accompanied by a decrease in SO2 concentrations measured in the air and in sulfate deposition recorded at monitoring sites operated by the National Atmospheric Deposition Program. Between 1990 and 2002 average SO2 concentrations in the atmosphere decreased by 54 percent. Wet sulfate deposition across the Northeast and Midwest declined by approximately 50 percent.
Between 1990 and 2002 NO5 emissions from power plants subject to the Acid Rain Program decreased from 6.7 million tons per year to 4.5 million tons per year. In 2000 the program achieved its goal of reducing emissions by at least two million tons; 8.1 million tons were originally predicted in 1990 to be emitted in the year 2000 without the program in place.
Decreased NO5 emissions have not resulted in uniformly lower levels of NO5 in the atmosphere or in deposits measured at recording stations. The EPA reports that concentrations of wet nitrates in the atmosphere generally remained constant between 1989 and 2002 across much of the country. In a few areas, concentrations actually increased. Progress for wet nitrate deposition was a little more promising. Large decreases in deposition were reported across the Northeast and the state of Michigan. Unfortunately, most of the Midwest and the mid-Atlantic regions showed little to no significant improvement.
Are Ecosystems Recovering?
Recovery Times
The EPA reports that ecosystems harmed by acid rain deposition can take a long time to fully recover even after harmful emissions cease. The most chronic aquatic problems can take years to be resolved. Forest health is even slower to improve following decreases in emissions, taking decades to recover from damage by acid deposition. Finally, soil nutrient reserves (such as calcium) can take centuries to replenish.
Recent Studies Show Mixed Results
According to the USGS in Trends in Precipitation Chemistry in the United States, 1983–1994: An Analysis of the Effects in 1995 of Phase 1 of the Clean Air Act Amendments of 1990, Title IV, rainwater tested at 109 test sites across the United States was less acidic in 1995 than in 1983, particularly along the Ohio River Valley and in the Mid-Atlantic region. SO5 had declined at 92 percent of the sites. The USGS attributed the improvement to the standards put in place by the Clean Air Act Amendments Title IV program. For nitrates, approximately as many sites showed decreased levels as reported increased levels. Overall, nitrate levels rose slightly, with the largest increases occurring in the western states.
In April 1999 NAPAP released findings from the study National Acid Precipitation Assessment Program Biennial Report to Congress: An Integrated Assessment. The study warned that, despite important strides in reducing air pollution, acid rain remained a serious problem in sensitive areas. The report provided additional evidence that acid rain is more "complex and intractable than was believed 10 years ago." Among the findings were the following:
- New York's Adirondack Mountain waterways suffer from serious levels of acid. Even though sulfur levels are declining, nitrogen levels there are climbing. The agency predicted that by 2040 about half the region's 2,800 lakes and ponds will be too acidic to sustain life.
- The Chesapeake Bay is suffering from excess nitrogen, which is causing algae blooms that suffocate other life forms.
- High elevation forests in Colorado, West Virginia, Tennessee, and southern California are nearly saturated with nitrogen, a key ingredient in acid rain.
- High elevation lakes and streams in the Sierra Nevadas, the Cascades, and the Rocky Mountains may be on the verge of "chronically high acidity."
The report concluded that further reductions in sulfur and nitrogen would be needed. The report also found, however, that the 1990 Clean Air Act Amendments have reduced sulfur emissions and acid rain in much of the United States. Some scientists believe that the problems associated with acid rain are theoretically reversible. That is, recovery is possible if a threshold of damage is not passed.
In March 2000 the EPA and the U.S. General Accounting Office (GAO) concluded in Acid Rain: Emissions Trends and Effects in the Eastern United States that some surface waters in New England harmed by acid rain were showing signs of recovery. However, ecosystems considered most severely affected—such as the Adirondacks—were not yet showing improvement. The GAO reported that acidified lakes in the Adirondack Mountains were taking longer to recover than lakes elsewhere and might not recover fully or at all without further reductions in acid deposition. Recovery was considered dependent on improving the nearby soil condition.
In early 2001 the EPA released a report on progress made by the United States and Canada on cross-border air pollution. The study, U.S.-Canada 2000 Air Quality Agreement Progress Report, is the fifth biennial report related to the 1991 agreement between the two countries. The report says that SO5 deposition was reduced by up to 25 percent between 1995 and 1998 over a large area of the eastern United States. Most of the reduction was in the Northeast, where many sensitive ecosystems are located. SO5 concentrations in lakes and streams decreased all over North America. Declines in nitrate concentrations were much smaller and rarer. Only one region, Vermont/Quebec, showed recovery as evidenced by decreasing acidity or increasing alkalinity.
In 2001 the Hubbard Brook Research Foundation released Acid Rain Revisited, which examined progress since 1990. The report concluded that acid rain was still a significant problem in the Northeast, despite declines in sulfur emissions. Researchers urged for tighter controls on emissions of NO5 and ammonia, two problems that have not been well addressed by the Acid Rain Program.
The report noted that many ecosystems in the northeast have reached or passed their tolerance for acid input, making recovery unlikely under the existing emissions reductions scheme. The researchers called for an additional 40 to 80 percent reduction in sulfur emission from electric utilities in addition to what is mandated now. Reductions at the 80 percent level are predicted to allow recovery of acidic streams to nonacidic status in approximately 20 to 25 years.
A Global Problem
Because of the transport properties of acid rain, it is not a localized problem. Emissions can originate hundreds of miles from where acid deposition occurs. Canadian authorities estimate that more than 30 percent of the acid rain that falls in Canada is due to U.S. emission sources.
In Europe pollutants are carried from the smokestacks of the United Kingdom over Sweden. In southwestern Germany many trees of the famed Black Forest are dying from the effects of acid rain transported to the region by wind. Germans have coined a word for the phenomenon, waldsterben (forest death).
Acid rain is a growing problem in Asia. According to the International Institute for Applied Systems Analysis, SO2 emissions in Asia are surpassing those in Europe and North America. In China acid rain is implicated in large die-offs in southwestern forests. A study by China's National Environment Protection Agency found that farmland is also affected by acid rain, so crops are at risk as well. According to Todd Johnson et al., in Clear Water, Blue Skies: China's Environment in the New Century, (Washington, DC: World Bank, 1997), the World Bank estimated annual forest and crop losses at $5 billion. Researchers believe it will be difficult to control pollution from China, the world's biggest consumer of coal, as that nation goes through an accelerated economic expansion that involves increased coal consumption.
Scientists estimate that about one-third of Japan's sulfur deposition comes from China. Atmospheric acidity levels are highest in the winter and early spring. During this time, huge air masses from continental Asia move to Japan, propelled by the prevailing monsoons. As in other countries,
Table 7.3
Public concern about acid rain, 2004
PLEASE TELL ME IF YOU PERSONALLY WORRY ABOUT THIS PROBLEM A GREAT DEAL, A FAIR AMOUNT, ONLY A LITTLE, OR NOT AT ALL. ACID RAIN?
| Great deal % | Fair amount % | Only a little % | Not at all % | No opinion % | |
| 2004 Mar 8–11 | 20 | 26 | 27 | 26 | 1 |
| 2003 Mar 3–5 | 24 | 26 | 27 | 21 | 2 |
| 2002 Mar 4–7 | 25 | 23 | 31 | 19 | 2 |
| 2001 Mar 5–7 | 28 | 28 | 26 | 16 | 2 |
| 2000 Apr 3–9 | 34 | 31 | 19 | 15 | 1 |
| 1999 Apr 13–14 | 29 | 35 | 23 | 11 | 2 |
| 1991 Apr 11–14 | 34 | 30 | 20 | 14 | 3 |
| 1990 Apr 5–8 | 34 | 30 | 18 | 14 | 4 |
| 1989 May 4–7 | 41 | 27 | 19 | 11 | 3 |
| SOURCE: "Please tell me if you personally worry about this problem a great deal, a fair amount, only a little, or not at all. Acid rain?," in Poll Topics and Trends: Environment, The Gallup Organization, Princeton, NJ, March 17, 2004 [Online] www.gallup.com [accessed March 30, 2004] | |||||
forests in Japan have experienced abnormally high death rates, particularly in stands of red pine and Japanese cedar. Japanese laws governing the emission of gases that acidify rain are among the strictest in the world. Nonetheless, Japan's rain is increasingly acidic. In Kawasaki, where NO5 levels are posted every day outside City Hall, the rain is sometimes as acidic as grapefruit juice.
In 2001 atmospheric scientists at Princeton University said that acid rain in Asia could triple over the next 30 years due to large expected increases in industrial emissions of NO5. Already, nearly 25 percent of China's NO5 emissions return to Earth in acid rain. Chinese emissions are blamed for more than 27 percent of NO5 acid rain in Japan and more than half in North Korea.
Public Opinion About Acid Rain
Every year the Gallup Organization polls Americans about their attitudes regarding environmental issues. The most recent poll was conducted in March 2004. As shown in Figure 1.8 in Chapter 1, acid rain ranked last among the environmental problems considered during the poll. Only 20 percent of respondents expressed a great deal of worry about acid rain. Table 7.3 shows a dramatic decline in concern about acid rain since the late 1980s. In 1989 Gallup found that 41 percent of respondents felt a great deal of concern about acid rain and 11 percent felt none at all. By 2004 only 20 percent of people polled were concerned a great deal about acid rain and more than a quarter of those asked expressed no concern about the acid rain issue.
|
This section contains 7,149 words (approx. 24 pages at 300 words per page) |


