|
This section contains 153 words (approx. 1 page at 300 words per page) |
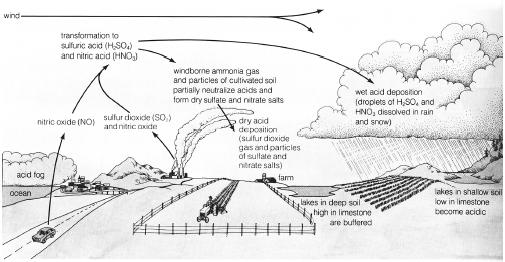 The basic mechanisms of acid deposition. (Illustration by Wadsworth Inc. Reproduced by permission.)
The basic mechanisms of acid deposition. (Illustration by Wadsworth Inc. Reproduced by permission.)
Acid precipitation from the atmosphere, whether in the form of dryfall (finely divided acidic salts), rain, or snow. Naturally occurring carbonic acid normally makes rain and snow mildly acidic (approximately 5.6 pH). Human activities often introduce much stronger and more damaging acids. Sulfuric acids formed from sulfur oxides released in coal or oil combustion or smelting of sulfide ores predominate as the major atmospheric acid in industrialized areas. Nitric acid created from nitrogen oxides, formed by oxidizing atmospheric nitrogen when any fuel is burned in an oxygen-rich environment, constitutes the major source of acid precipitation in such cities as Los Angeles with little industry, but large numbers of trucks and automobiles. The damage caused to building materials, human health, crops, and natural ecosystems by atmospheric acids amounts to billions of dollars per year in the United States.
|
This section contains 153 words (approx. 1 page at 300 words per page) |


