 pH scale. (McGraw-Hill Inc. Reproduced by permission.)
pH scale. (McGraw-Hill Inc. Reproduced by permission.)
The following sections of this BookRags Literature Study Guide is offprint from Gale's For Students Series: Presenting Analysis, Context, and Criticism on Commonly Studied Works: Introduction, Author Biography, Plot Summary, Characters, Themes, Style, Historical Context, Critical Overview, Criticism and Critical Essays, Media Adaptations, Topics for Further Study, Compare & Contrast, What Do I Read Next?, For Further Study, and Sources.
(c)1998-2002; (c)2002 by Gale. Gale is an imprint of The Gale Group, Inc., a division of Thomson Learning, Inc. Gale and Design and Thomson Learning are trademarks used herein under license.
The following sections, if they exist, are offprint from Beacham's Encyclopedia of Popular Fiction: "Social Concerns", "Thematic Overview", "Techniques", "Literary Precedents", "Key Questions", "Related Titles", "Adaptations", "Related Web Sites". (c)1994-2005, by Walton Beacham.
The following sections, if they exist, are offprint from Beacham's Guide to Literature for Young Adults: "About the Author", "Overview", "Setting", "Literary Qualities", "Social Sensitivity", "Topics for Discussion", "Ideas for Reports and Papers". (c)1994-2005, by Walton Beacham.
All other sections in this Literature Study Guide are owned and copyrighted by BookRags, Inc.
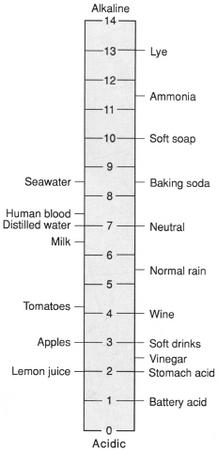
A measure of the acidity or alkalinity of a solution based on its hydrogen ion (H+) concentration. The pH of a solution is the negative logarithm (base 10) of its H+ concentration. Since the scale is logarithmic, there is a tenfold difference in hydrogen ion concentration for each pH unit. The pH scale ranges from 0 to 14 with 7 indicating neutrality ((H+)= (OH-)). Values above 7 indicate progressively greater alkalinity, while values below 7 indicate progressively increasing acidity.
 pH scale. (McGraw-Hill Inc. Reproduced by permission.)
pH scale. (McGraw-Hill Inc. Reproduced by permission.)